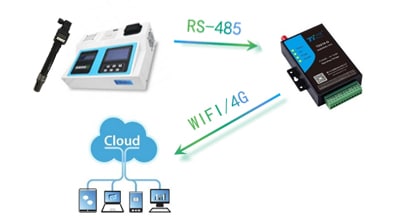
Conductivity TDS Meter & Sensor
Apure is a recognized world leader for reliable conductivity equipment, providing credible and repeatable measurement solutions for maintaining and controlling even the most demanding process applications. Used to measure TDS and electrical conductivity of water or solutions; used in hydroponics, aquaculture, aquaponics, and freshwater systems to monitor quantities of nutrients, salts, or impurities.
Apure offers conductivity meters and sensors suitable for measuring the two most common conductivity measurement methods: contacting and inductive (also known as toroidal or electrodeless). Our conductivity analyzers provide high precision measurement and are built to withstand the harshest applications, requiring minimum maintenance and helping you achieve increased efficiency and reduced operating costs.
What is electrical conductivity?
Conductivity, simply put, is the ability of a solution to conduct an electric current. It reflects the concentration of ions in a solution. The higher the concentration of ions, the greater the ability of the solution to conduct electricity and the greater the conductivity.
How a conductivity meter works?
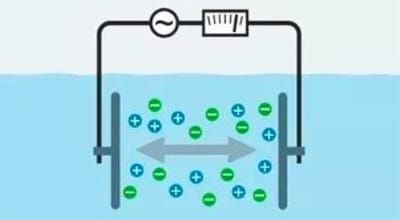
A conductivity meter is an instrument that measures the ability of a solution to conduct electricity. Its principle of operation is based on Ohm’s law, which states that current is proportional to voltage and inversely proportional to resistance.
- Role of the electrodes: The electrodes of the conductivity meter are immersed in the solution to be measured, forming a conductive circuit.
- Generation and Measurement of Current: The instrument applies a weak alternating current to the electrodes, and the ions in the solution move in a directional manner under the action of the electric field, forming a current. The instrument measures the magnitude of this current.
- Calculation of Conductivity: The conductivity of a solution is calculated using Ohm’s law from the measured current, the applied voltage, and the geometry of the electrode (electrode constant).
Relationship between conductivity and solution properties.
- Ion Concentration: The higher the ion concentration, the more conductive the solution is and the greater the conductivity.
- Ion species: Different ions have different mobilities, which also affect the conductivity of a solution.
- Temperature: As the temperature increases, the ions move faster and the conductivity of the solution usually increases.
Types of conductivity sensors and how they work?
The conductivity sensor is the core component of the conductivity meter, which can be categorized into the following types according to the different measurement principles:
Electrode Type Sensor
- Two-electrode: The most common type, consisting of a pair of electrodes, the working principle is as described above.
- Four-electrode type: Adopting four-electrode structure can effectively reduce the polarization effect and improve the measurement accuracy.
Electromagnetic Induction Sensor
- Utilizes the principle of electromagnetic induction without direct contact with the solution, and is suitable for corrosive or viscous liquids.
- An alternating magnetic field is generated inside the sensor, and when a conductive liquid flows through it, an eddy current is induced in the liquid, and the conductivity is calculated by measuring the size of the eddy current.
Ultrasonic Sensor
- Measurement utilizing the relationship between the propagation speed of ultrasonic waves in a liquid and the conductivity of the liquid.
- The conductivity of the liquid is calculated by measuring the propagation time of the ultrasonic waves in the liquid.
Applications
- Water Quality Monitoring: Monitoring the purity and pollution level of drinking water, industrial water, wastewater.
- Industrial process control: Monitoring the concentration of electroplating solution, acid and alkali solution to control the production process.
- Food industry: Monitoring salinity and sugar level during food processing.
- Environmental monitoring: Monitoring of soil and groundwater pollution.
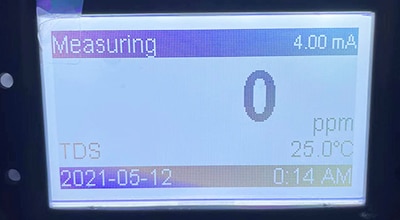
What is TDS and how does a TDS meter work?
TDS (Total Dissolved Solids) refers to the total amount of all solids dissolved in water. These solids can be inorganic salts (e.g. calcium, magnesium, sodium, etc.), organics, and other impurities.The higher the TDS value, the more dissolved solids are in the water.
The TDS meter measures TDS indirectly by measuring the conductivity of the water. The conductivity of water is directly proportional to the concentration of ions in the water, and the concentration of ions is closely related to the TDS value.
Factors affecting conductivity measurement
- Temperature: Temperature changes affect the conductivity of a solution, so conductivity meters usually have a temperature compensation function.
- Electrode contamination: Electrode surface adsorbed impurities or corrosion, will affect the measurement results, need to be cleaned regularly.
- Properties of the solution: The viscosity and dielectric constant of the solution will affect the conductivity.