pH ORP Controller & Sensor
Obtaining accurate pH and ORP measurements from your analyzer and interpreting this data correctly is critical to ensuring product quality and making accurate predictions of sensor lifetime.
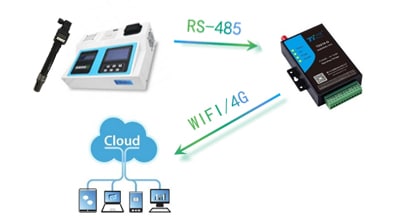
Apure’s pH and ORP measurement solutions include 2-wire and 4-wire analyzers featuring real-time online sensor diagnostics to provide reliable results. Our pH and ORP analyzers use the latest analyzer sensor technology to improve predictive maintenance and reduce the time and costs associated with sensor maintenance and replacement, optimizing your OPEX.
What is a pH and ORP controller?
pH and ORP controllers are automated devices used to monitor and control the acidity and alkalinity (pH) and oxidation reduction potential (ORP) of liquids.
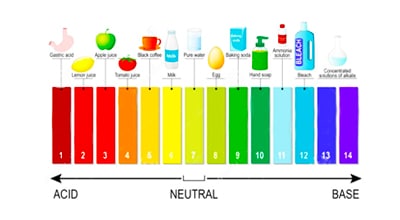
- pH value: indicates the degree of acidity or alkalinity of a solution. The lower the pH value, the more acidic the solution; the higher the pH value, the more alkaline the solution.
- ORP value: measures the strength of redox reactions in a solution, the higher the ORP value, the more oxidizing the solution is.
Working Principle of pH and ORP Controller
- Measurement: The pH and ORP electrodes on the controller continuously measure the pH and ORP values of the liquid.
- Comparison: The controller compares the measured values with the set target values.
- Control: If there is a difference between the actual value and the target value, the controller will automatically start the corresponding equipment (e.g., acid pump, alkali pump, etc.) to adjust the pH or ORP value of the liquid until the set target is reached.
Types of pH Controllers
- Analog pH Controller:Simpler and cheaper, but may have limitations in terms of functionality and accuracy.
- Digital pH controllers: offer more advanced features such as data logging, alarms and remote control.
- Wi-Fi enabled pH controllers: allow remote monitoring and control via smartphone or computer.
What is PH/ORP Sensor?
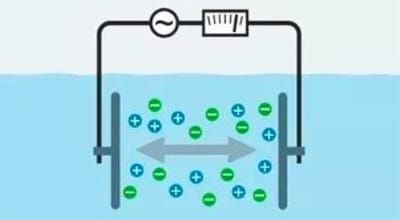
PH/ORP sensor is an important part of PH/ORP controller, which is used to directly feel the PH and ORP values in the measured solution and convert them into electrical signals for output to the controller for processing. The sensor is generally composed of four parts: sensitive element, conversion element, conversion circuit and auxiliary power supply, and is characterized by miniaturization, digitization and intelligence.
pH ORP Sensor Types
Classification by sensor type
- Flexible: this sensor is designed to be flexible, easy to install and replace, and is suitable for a variety of measurement environments.
- Insertion type: Insertion type sensors are usually used in scenarios where measurement needs to be made deep inside the liquid, such as industrial water treatment, environmental monitoring and other fields. They have a robust structure and long cables for stable operation in liquids.
- Hygienic: Hygienic sensors pay particular attention to cleaning and sanitizing and are typically used in industries that require high hygiene standards, such as pharmaceutical, food and beverage. These sensors are made from materials that are easy to clean and sanitize, such as 316SS (stainless steel) and PVDF (polyvinylidene fluoride).
- Submerged: Submerged sensors are fully or partially submerged in the liquid to be measured and are capable of continuously and consistently monitoring the pH and ORP of the liquid. They usually have longer cables and sturdy housings to adapt to a variety of harsh measurement environments.
According to the electrode material classification
- General-purpose glass electrodes: This is one of the most common electrode materials, applicable to most water quality monitoring scenarios. They have good stability and measurement accuracy.
- Hydrofluoric acid-resistant glass electrode: this electrode is able to resist the corrosion of hydrofluoric acid, which is especially suitable for industrial wastewater treatment containing hydrofluoric acid and other fields.
- Platinum electrodes: Platinum electrodes are used for ORP measurements and have good conductivity and stability. They are usually used in conjunction with different diaphragm types to adapt to different measurement environments.
According to the technical characteristics of the classification
- Digital sensors: digital sensors built-in smart chip and signal processing circuitry, able to convert the measurement signal directly into a digital signal output. This sensor has the advantages of high precision, high stability and easy integration.
- Analog sensors: Analog sensors output analog signals (such as voltage or current signals), which need to be processed by an analog/digital converter (ADC) to obtain digital results. This type of sensor, while less costly, may be slightly inferior to digital sensors in terms of accuracy and stability.
Applications
- Water Treatment: Control pH and ORP of drinking water, swimming pool water, industrial wastewater.
- Electroplating: Control the pH and ORP value of electroplating solution to ensure the quality of plating layer.
- Food industry: Control the pH value during food processing to ensure the taste and safety of food.
- Pharmaceutical industry: Control the pH and ORP value in the process of drug production to ensure the quality of drugs.
- Aquaculture: Control the pH and ORP value of aquaculture water to provide a suitable growing environment for aquatic animals.