Salinity Meter & Sensor
Salinity Meter is an instrument used to quickly determine the concentration or refractive index of saline solution by weight. It usually consists of sensors, measuring circuits and data processing devices. Salinity sensor has two kinds of electrode type and induction type.
Salinity sensors are used to measure the salinity of liquids and solutions and are capable of measuring the entire range from 24 to 52,000 ppm (parts per million). Salinity is the sum of all non-carbonate salts dissolved in water and is usually expressed in parts per thousand (1 ppm = 1000 mg/L). Salinity is an important measurement in seawater. The salinity level in seawater is fairly constant at about 35 ppm (35,000 mg/L).
How does a salinity sensor work?
Apure industrial salinity meter and sensor measures the ability of a solution to conduct a current between two electrodes. In a solution, current flows through ion transport; therefore, an increase in the concentration of ions in the solution will result in a higher conductivity value.
A salinity sensor actually measures conductance, defined as the reciprocal of resistance. Even though the salinity sensor is measuring conductivity, we are interested in finding the conductivity of the solution. A potential difference is applied to the two probe electrodes in the salinity sensor. The current generated is proportional to the conductivity of the solution. This current is converted to a voltage.
An alternating current is supplied to prevent complete migration of ions to both electrodes. With each cycle of the AC current, the polarity of the electrodes is reversed, which in turn reverses the direction of ion flow. This very important feature of the salinity sensor prevents most electrolysis and polarization from occurring at the electrodes. As a result, the solution in which the conductivity is being measured is not contaminated. It also greatly reduces the formation of redox products on the electrode.
- Conductivity Measurement: A salinity sensor typically consists of a pair of electrodes that generate an electric current when immersed in a solution. The magnitude of the current is proportional to the conductivity of the solution, which in turn is related to the concentration of ions in the solution.
- Signal Conversion: The sensor converts the measured conductivity signal into a readable value such as millisiemens per centimeter (mS/cm) or other units.
- Temperature Compensation: Temperature affects the conductivity of a solution, so most salinity sensors are temperature-compensated to ensure accurate measurements.
Salinity Sensor Type
- Electrode type: Uses one or more pairs of electrodes to directly measure the solution conductivity.
- Inductive: electromagnetic induction principle, without direct contact with the solution, suitable for corrosive or easily contaminated media.
- Refractive: through the measurement of the refractive index of the solution to deduce the salt concentration, suitable for high concentration salt solution measurement.
Electrode calibration instructions
Start calibration
First step
- Clean and dry the electrode, put it in the standard solution
- Send command 01 02 C4 0B
- After reading the measured value to be stabilized, calculate the conductivity constant, conductivity constant=standard solution value /current measured value
- For example, the electrode is placed in the 1.413ms/cm standard solution and the current measured value of the electrode is 1. 450ms/cm
- Then the conductivity constant=1. 413/1. 450=0.97448
- To write data to the OXOA register 0.97448
- Send command:01 10 00 0A 00 02 04 77 85 3F 79 A9 9F;
End of calibration
Salinity sensor installation
- Conductivity electrode is recommended to be instal the flow cell for more stable and accurate measurement
- If installing electrode in the pipe, the right angle should be 15’m165
- Installation method
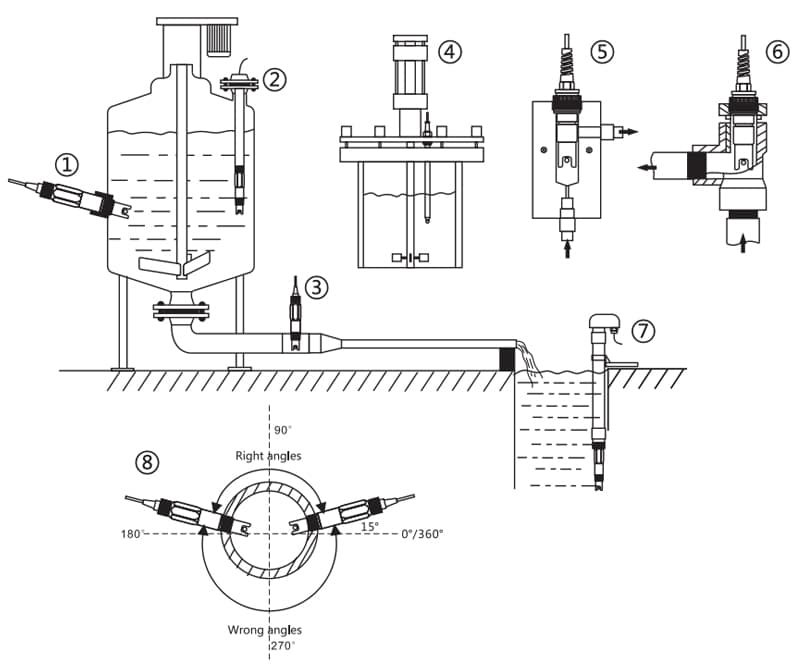
- side wall installation
- top flange installation
- ipe installation
- top installation
- flow cell installation
- pipe Installation
- immersion installation
- anales in pipe installation
Maintenance and storage
- The organic dirt on the electrode can be cleaned with warm water containing detergent or alcohol. After cleaning the electrode, it should be dried by soft tissue.
- When the electrode is stored. it shall be dried and stored.
- Cable connector must be kept clean and free from moisture or water.
Applications
- Aquaculture: To monitor the salinity change of seawater or freshwater aquaculture pools to ensure the stability of water quality.
- Food industry: Used to measure the salt concentration during food processing, such as soy sauce, pickled food, etc.
- Chemical industry: monitoring the concentration of salt solution in the production process to control the reaction process.
- Environmental monitoring: Measure the salt content in water and soil to assess the environmental quality.
- Desalination: Monitor the salinity of desalinated water to ensure that the effluent water quality meets the standards.




































