A conductivity probe is a key sensor device used to measure the conductivity of a solution and has a wide range of applications and importance.
How do conductivity probes work?
Concept and definition of conductivity
Conductivity is a physical quantity that describes the ability of a solution to conduct an electric current and is usually expressed as conductivity (σ) in Siemens per meter (S/m). It expresses the ability of a solution to conduct current per unit volume, i.e., the effect of the content and activity of ionized substances in the solution on the conductivity. A higher conductivity indicates a greater concentration of ions in the solution and a greater ability to conduct current.
Conductivity probe composition and working principle
A conductivity probe usually consists of two electrodes, one of which acts as the transmitting electrode and the other as the receiving electrode. When a constant current is passed through the conductivity probe, ions in the solution cause the current to flow and create a potential difference between the probes. By measuring this potential difference, the conductivity of the solution can be determined.
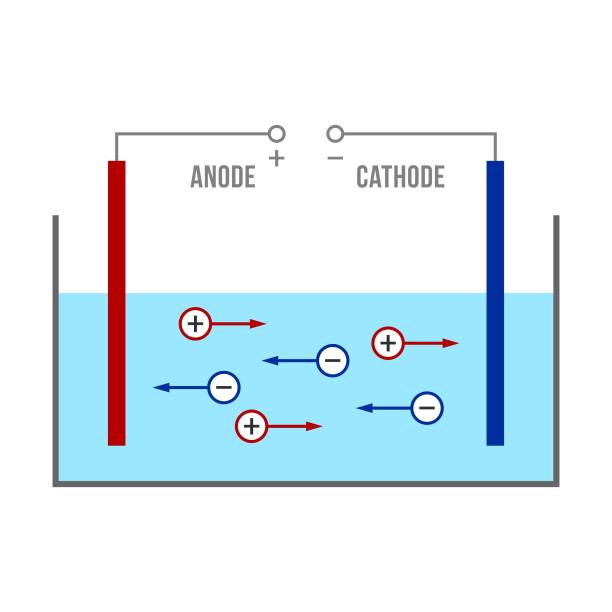
There is a direct relationship between conductivity and the concentration of ions in the solution. The higher the concentration of ions in a solution, the higher its conductivity, because the ions can conduct electricity more efficiently. For this reason, conductivity probes are often used to estimate the concentration of ions in solution, both cations (e.g., sodium ions, calcium ions) and anions (e.g., chloride ions, sulfate ions). This relationship is important for water quality monitoring, chemical reaction control, and solution analysis in industrial processes.
Application areas of conductivity probes
Conductivity probes have a wide range of applications in many different fields, including but not limited to:
- Water quality monitoring: Used to monitor conductivity in water bodies such as tap water, wastewater treatment plants, rivers and lakes to assess the salinity, ionic content and pollution level of the water.
- Industrial production: used in chemical, pharmaceutical, food and beverage industries to monitor the concentration, purity and ionic content of solutions during the production process to ensure product quality and production safety.
- Laboratory research: for analysis of solutions, control of chemical reactions and acquisition of experimental data, which is used in the fields of chemistry, biology and environmental science.
- Agricultural field: Used for soil salinity and ion concentration measurement to help farmers apply fertilizers and manage soil nutrients rationally.
- Environmental monitoring: for monitoring salinity, pollutant concentration and water quality changes in water bodies such as seawater, groundwater, rivers and lakes to protect the health of the environment and ecosystems.
- Life science research: In cell culture and biological experiments, it is used to monitor the ion concentration of solutions such as culture medium and saline to maintain the stability of the cell growth environment.
Advantages of conductivity probes
- Real-time monitoring: The conductivity probe measures the conductivity of a solution in real time, providing instant data feedback that helps to identify and solve problems promptly.
- Non-destructive: The measurement process of the conductivity probe does not require the destruction of the sample, it only needs to immerse the probe in the solution to carry out the measurement, without affecting the structure and properties of the sample.
- Wide applicability: The conductivity probe is suitable for various types of solutions, including water, acid, alkali, salt solutions, etc., and has a wide range of applicability to cover a wide range of ion concentrations.
- High sensitivity: The conductivity probes have high responsiveness to small ion concentration changes and can accurately detect ion concentration changes in solution.
- Automation and Integration: The conductivity probe can be integrated with automation systems and data acquisition systems to realize automated monitoring and data logging, improving work efficiency and data quality.
- Easy maintenance: Conductivity probes are simple and easy to use, and usually require only regular cleaning and calibration to maintain stable performance.
Different types of conductivity probes
Different types of conductivity probes can be categorized into the following main types based on their operating principles, structures and application scenarios:
- Conductive conductivity probes: Conductive conductivity probes measure the conductivity of a solution by conducting current between two electrodes. This type of probe usually consists of two electrodes, one of which acts as the transmitting electrode and the other as the receiving electrode, and determines the conductivity of a solution by measuring the flow of current. Conductivity probes are suitable for various types of solutions and are characterized by simplicity and practicality.
Apure KDM EC Electrical Conductivity Sensor is a conductivity sensor for use in acid/base/salt solutions, chemical processes, and industrial processes.

- Inductive conductivity probes: Inductive conductivity probes utilize the principle of electromagnetic induction to measure the conductivity of a solution. When an alternating electric field is applied to a solution, the ions in the solution generate an electric current, which produces an induced voltage. By measuring the magnitude of the induced voltage, the conductivity of the solution can be determined. Inductive conductivity probes are typically used for high purity water and low conductivity solutions.
- Resonant conductivity probes: Resonant conductivity probes utilize the capacitance and inductance between the electrodes to form a resonant circuit that determines the conductivity of a solution by measuring the resonant frequency of the circuit. This type of probe is typically used for precise measurements and high sensitivity applications such as laboratory research and monitoring of high purity solutions.
- Needle conductivity probes: A needle conductivity probe is a type of conductivity probe in which an electrode is placed in the solution for measurement. It typically has a smaller probe size and sensitivity and is suitable for small sample measurements and high-precision applications such as laboratory research and medical diagnostics.
Summary
As a key measurement device, conductivity probe plays an important role in various fields. It can monitor the ion concentration and purity in solution in real time, providing reliable data support for quality control, environmental monitoring, experimental research, medical diagnosis and agricultural applications. With the continuous development of technology and the expansion of application scope, the importance and application prospect of conductivity probes will be more extensive and far-reaching.
Apure not only provides conductivity probes, but also conductivity meters and sensors. In addition to devices for monitoring conductivity parameters, there are also devices for monitoring pH, residual chlorine, dissolved oxygen, turbidity, salinity, ozone, corrosion rate, multi-parameter, chlorophyll, blue-green algae, chemical oxygen demand, ammonia, nitrate and nitrite. Please contact us for more information.
