Reference electrodes play a crucial role in electrochemical measurements. As a stable reference point for the potential, the reference electrode not only ensures the accuracy and repeatability of the measurement, but also provides a stable potential environment that enables the electrode under test to respond accurately to the electrochemically active substances in the solution to be measured.
Principle and function of reference electrodes
Reference electrodes play a key role in electrochemical measurements, and their basic principle involves three aspects. First, the reference electrode provides a stable potential reference point that serves as a standard for the potential in the solution to be measured. This stabilized potential can help ensure the accuracy and repeatability of the measurement results because it allows the measuring device to be compared to a uniform reference point, thus eliminating the effect of variations in potential on the measurement results.
Secondly, reference electrodes also ensure potential stability in the measurement system. By comparing it to the electrode to be measured in the solution being measured, the reference electrode helps to identify and compensate for potential drifts or disturbances that may be present in the measurement system, thus ensuring the accuracy of the measurement results.
Finally, the reference electrode also serves as a standardizer in electrochemical experiments. Its stable potential environment not only ensures the accuracy of the measurement results, but also improves the repeatability and comparability of the experiments, thus promoting the progress and application of electrochemical research. In summary, the role of reference electrode in electrochemical measurements is indispensable, which not only provides a stable potential reference, but also ensures the stability of the measurement system and promotes the standardization and comparability of experiments.
Working mechanism of reference electrodes
The working mechanism of a reference electrode involves several aspects such as electrode reaction and ion transport. First, a reference electrode usually consists of an electrode and a reference solution. Chemicals in the reference solution (e.g., potassium chloride) react with the electrode to produce a stable potential. This reaction causes a fixed potential to form on the surface of the electrode, which serves as a reference point.
Second, the electrode reaction in a reference electrode is usually reversible. This means that the electrode reaction can go back and forth across the electrode surface without irreversible chemical changes. This reversibility ensures the stability and reliability of the reference electrode, allowing it to provide a stable potential reference.
In addition, ion transport is one of the key factors in the operation of a reference electrode. In the reference solution, ions are transported in the electrolyte solution and react with the electrode surface, thus maintaining the potential stability of the electrode surface. Good ion transport ensures a fast response and stable potential of the reference electrode, thus improving the accuracy and reliability of the measurement.
Types of reference electrodes
Silver/Silver potassium chloride electrode
- Features: The silver/silver potassium chloride electrode is one of the most common and widely used reference electrodes. It consists of a silver wire electrode and a saturated solution of silver potassium chloride to provide a stable potential reference through redox reactions between silver/silver ions.
- Applicable Scenarios: Suitable for a wide range of electrochemical measurements and experiments, including acid-base titration, electrochemical deposition, potentiometric titration, and so on.
- Advantages: High stability, fast response time, applicable to a wide range of electrochemical measurements.
- Disadvantages: susceptible to contamination by silver ions, requires regular cleaning and regeneration.
Apure e-201 Lab Glass pH Electrode is the glass electrode and reference electrode together molded case rechargeable type composite electrode, PH measurement element, is used to measure the PH of aqueous solution (hydrogen ion activity).
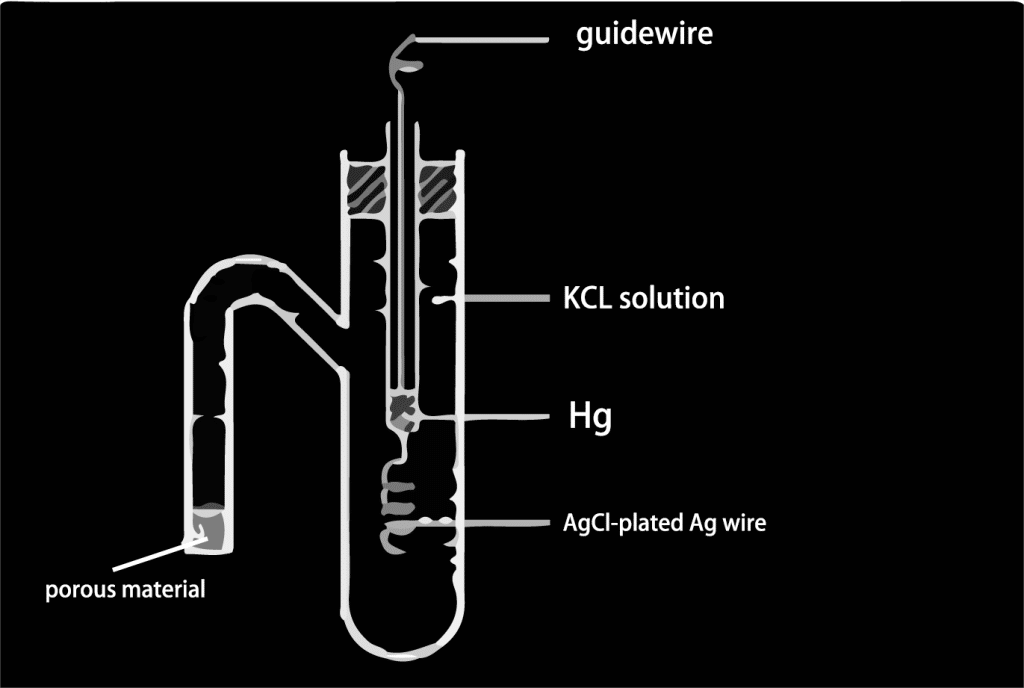
Saturated calomel electrode
- Characteristics: The saturated calomel electrode is another common type of reference electrode, which consists of liquid mercury in equilibrium with its vapor-phase mercury/mercury sulfide electrode, and has very high stability and low electrode response impedance.
- Scenario: Suitable for experiments requiring high electrode response speed, such as fast electrochemical cyclic voltammetry, polarization scanning, etc.
- Advantages: high stability, fast response speed, suitable for rapid electrochemical experiments.
- Disadvantages: the use of the process may produce mercury vapor, there is a safety hazard, and is not applicable to oxidizing media.
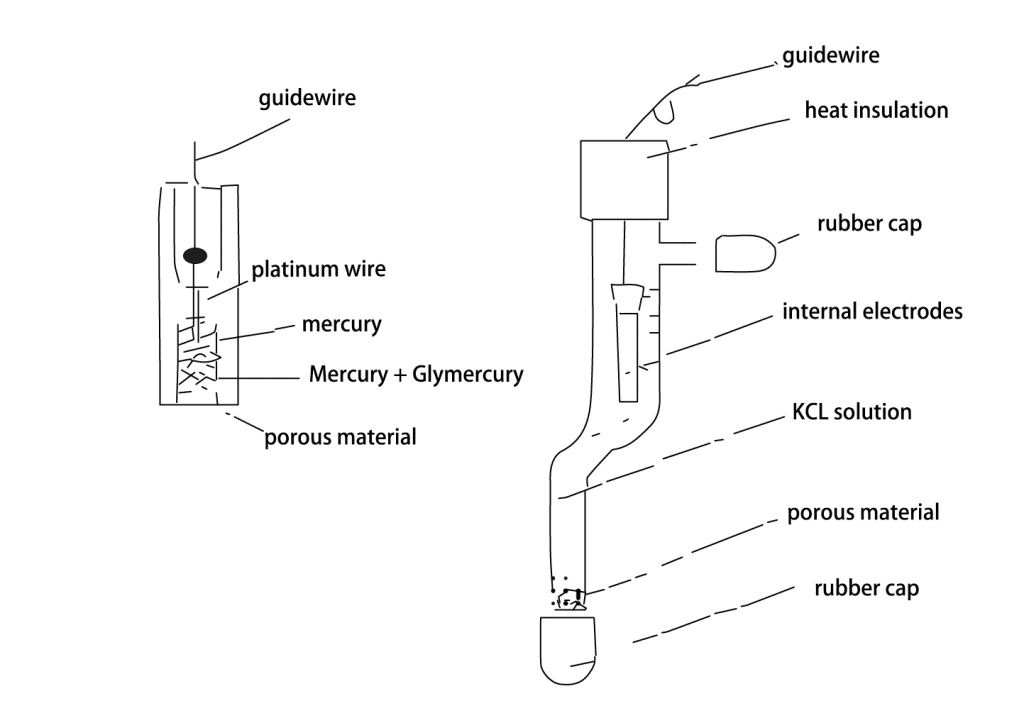
Silver/Silver silver chloride electrode
- Features: Silver/silver silver chloride electrode is similar to silver/silver potassium chloride electrode, but the reference solution in silver chloride electrode is saturated silver silver chloride solution, which has higher electrode stability and wider working temperature range.
- Scenario: suitable for electrochemical measurements under high temperature or extreme environmental conditions, such as fuel cells, high temperature electrolysis, etc.
- Advantages: High stability, suitable for measurements under high temperature or extreme environmental conditions.
- Disadvantages: More expensive than silver/silver potassium chloride electrodes and requires the use of special reference solutions.
The following factors need to be considered when selecting a reference electrode:
- Experiment type and purpose: Different types of experiments may have different requirements for electrode stability, response speed, etc.
- Working environment: Consider the temperature, pressure and other conditions at which the experiment is conducted and choose a suitable reference electrode.
- Economic cost: silver/silver potassium chloride electrodes are relatively inexpensive, while saturated calomel electrodes are more expensive.
- Safety considerations: If the experimental environment has strict restrictions on mercury vapor, then saturated calomel electrodes should be avoided.
The Importance of Proper Use of Reference Electrodes
First, proper reference electrode selection and preparation is essential. The appropriate type of reference electrode should be selected according to the experimental needs and conditions, and it should be ensured that both the reference electrode and the electrode to be measured are in a clean state without contamination or deposits.
Secondly, the operating procedures should be strictly followed when installing and calibrating the reference electrode. Ensure that the reference electrode is fully immersed in the solution to be measured and calibrate the reference electrode using a standard solution. At the same time, avoid air bubbles and temperature changes to interfere with the measurement results.
Maintain the stability and accuracy of the reference electrode at all times during the experimental operation. The electrode to be measured and the reference electrode should be immersed in the solution to be measured at the same time, and ensure that the measurement environment is stable to prevent the influence of external factors on the measurement results.
Finally, the reference electrode should be cleaned and stored in time after the experiment, and its condition should be checked and maintained and calibrated regularly. Only through proper use and maintenance can we ensure that the reference electrode always provides an accurate and reliable potential reference, thus ensuring the accuracy and repeatability of the experimental results.
Application of reference electrodes in experiments
Reference electrodes are widely used in different fields and experiments. In electrochemical analysis, reference electrodes are commonly used to measure potentials and monitor changes in electrochemical parameters during reactions, such as in cell preparation, corrosion studies and electrochemical synthesis. In corrosion studies, reference electrodes can be used to measure the corrosion potential of the metal surface, evaluate the corrosion rate and corrosion behavior, so as to provide a reference basis for the corrosion prevention design of materials.
In addition, in the field of environmental monitoring, reference electrodes are also widely used in water quality monitoring, soil analysis and atmospheric pollution monitoring, etc. They are used to measure key parameters such as pH value and redox potential, which help to monitor the chemical changes and pollution level in the environment so as to protect the environment and human health.
Summary
By providing an accurate and reliable potential reference, reference electrodes can ensure the accuracy and repeatability of experimental results, providing important data support for scientific research, engineering design and environmental protection.
Apure offers water quality monitoring analyzers and electrodes equipped with various types of electrodes, as well as temperature transmitters, pressure transmitters, flow meters and level meters. Please feel free to contact us if you need any assistance.
