Ion-selective electrodes are one of the crucial instruments in chemical analysis, and they play a key role in detecting the concentration of specific ions in a solution.
Composition and basic principle of ion selective electrode
Ion-selective electrodes usually consist of three main parts: the electrode body, the reference electrode, and the ion-selective membrane. The electrode body contains the measuring part of the electrode and is usually a tiny glass sphere or glass tube filled with an electrolyte solution containing specific ions. The reference electrode is used to provide a stable potential reference to ensure the accuracy and repeatability of the measurement. The ion-selective membrane, located on the surface of the electrode body, is a semi-permeable membrane that selectively reacts chemically with the target ion.
Ion-selective membranes selectively interact with target ions through their special chemical properties. This interaction causes a change in the concentration of ions on the membrane, leading to a corresponding change in the electrolyte within the electrode body, resulting in a change in potential. When the target ion reacts with the ion-selective membrane, the potential inside the electrode body changes and this change can be measured indirectly by measuring the potential of the electrode to measure the concentration of the target ion. Thus, an ion-selective electrode can determine the concentration of a specific ion in solution by measuring the electrode’s potential change
Nernst equation versus ion concentration
The Nernst equation plays a key role in ion-selective electrodes by describing the relationship between the electrode potential and the concentration of ions measured by the electrode. The general form of the Nernst equation is given below:
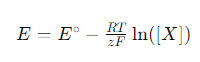
Where E is the potential of the electrode, E∘ is the standard electrode potential, R is the ideal gas constant, T is the absolute temperature, z is the charge of the ion, F is the Faraday constant, and [X] is the concentration of the target ion in solution.
Ion-selective electrodes utilize the Nernst equation to measure the concentration of specific ions in solution. When an ion-selective membrane reacts with a target ion, the state of charge on the surface of the membrane changes, resulting in a change in potential within the electrode body. The relationship between this change in potential and the concentration of the target ion follows the Nernst equation. By measuring the potential change of the electrode, the Nernst equation can be utilized to invert the concentration of the target ion in the solution.
Therefore, ion-selective electrodes utilize the relationship between the Nernst equation and the concentration of the target ion in solution to achieve the measurement of ion concentration. According to the Nernst equation, the potential of the electrode increases and decreases as the concentration of the target ion increases and vice versa, allowing for accurate measurement of the concentration of specific ions in solution.
Principles and applications of specific ion-selective electrodes
Common ion-selective electrodes include pH electrodes, potassium ion electrodes, chloride ion electrodes, and so on. They each work based on different principles and play an important role in practical applications.
- pH electrode: The pH electrode is a key instrument for measuring the acidity or alkalinity of a solution. The basic principle is to utilize the glass membrane to react with the hydrogen ions in the solution to produce a potential difference, which is directly proportional to the pH value of the solution. This potential is proportional to the pH value of the solution, so the pH value of the solution can be determined by measuring the potential. E-201 Lab Glass pH Electrode is widely used in the fields of water quality monitoring and biomedical research.


- Potassium ion electrode: A potassium ion electrode is used to measure the concentration of potassium ions in a solution. Its working principle is based on the selective reaction of the ion-selective membrane to potassium ions, which is proportional to the potential change of the potassium ion concentration in the solution. PMI-201X Potassium Ion Selective Electrode is of great significance in soil analysis, agricultural production, and other fields.

- Chloride ion electrode: The chloride ion electrode is used to measure the concentration of chloride ions in solution. Its working principle is similar to that of the potassium ion electrode, utilizing the selective reaction of the ion-selective membrane to chloride ions to produce a potential change proportional to the concentration of chloride ions. CLI-406 Chloride Ion Selective Electrode is widely used in water quality monitoring, swimming pool water treatment, and other applications.


These ion-selective electrodes can accurately measure the concentration of specific ions in solution by measuring changes in the potential of the electrode, providing reliable data support for experiments and production in a variety of fields.
Advantages and disadvantages of ion-selective electrodes
Advantages:
- High selectivity: the ion-selective electrode can specifically detect the target ion and is insensitive to other ions, with high selectivity.
- High sensitivity: Ion selective electrode has high response sensitivity to the concentration change of target ions, and can quickly and accurately detect small concentration changes.
- Direct measurement: The Ion selective electrode can be measured directly in the solution, without complex pre-treatment steps, easy to operate.
- Real-time monitoring: Ion-selective electrodes can monitor the concentration change of target ions in the solution in real-time, timely feedback data, convenient for real-time control and adjustment.
Disadvantages:
- Interference: ion-selective electrodes are susceptible to interference from other ions during the measurement process, which may lead to deviations in the measurement results.
- Limited scope of application: different types of ion-selective electrodes have a limited scope of application, and it is necessary to select the appropriate electrode type according to the specific measurement requirements.
- High maintenance requirements: Ion-selective electrodes require regular maintenance and calibration to maintain their sensitivity and accuracy, which increases the cost and workload.
- Higher cost: Ion-selective electrodes are more expensive to manufacture and relatively expensive to purchase and maintain, which may be less economical and practical for some laboratories and production scenarios.
Summary
The ion-selective electrode is a key instrument for chemical analysis, which measures the concentration of target ions in solution using the Nernst equation through the interaction of a specific selective membrane with the target ions. It has the advantages of high selectivity, high sensitivity, and direct measurement, and plays an important role in the fields of water quality monitoring, biomedical research, and food processing. With the continuous progress of science and technology, the application fields of ion-selective electrodes will be further expanded to provide more accurate and rapid data support for chemical analysis and experimental research, which is expected to bring a broader development prospect in the fields of environmental protection and medical diagnosis.
Apure provides not only ion-selective electrodes, but also other water quality analyzers, including residual chlorine controllers, dissolved oxygen controllers, conductivity controllers, and so on, Apure will work together with you to create a better environment, to provide better water quality and environmental services, if you need, please consult us.
