Ammonia & Ammonium Sensor
Ammonium (NH 4 + ) – or its uncharged form, ammonia (NH 3 ) – is a form of nitrogen that aquatic plants can take up and bind to proteins, amino acids and other molecules. High concentrations of ammonium can promote the growth of algae and aquatic plants. Bacteria can also convert high ammonium salts to nitrate (NO 3 -) during nitrification, which reduces dissolved oxygen.
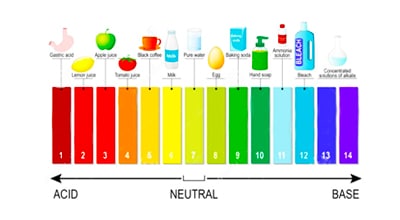
Ammonia in water is either non-ionic ammonia or ammonium ions. Usually, the value reported is the sum of the two forms and is reported as total ammonia or simply – ammonia. The relative proportions of the two forms present in water are strongly influenced by the pH value.
Non-ionic ammonia is the toxic form and dominates at high pH values. NH 4+ ions are relatively non-toxic and dominate at low pH values. Typically, less than 10% of ammonia is in the toxic form at pH values less than 8.0 pH units. This percentage increases significantly with increasing pH. The balance between NH 3 and NH 4 + is also affected by temperature. At any pH, more toxic ammonia is present in colder water.
Nitrogen exists in many compounds and forms. In municipal wastewater, it is encountered mainly as a waste product in the form of urea, which has been partially converted to ammonium nitrogen by ammonification. In an aeration tank, the initial step of nitrification consists in the oxidation of the nitrogen present in the wastewater to nitrate by nitrite, which requires oxygen.
For fish, ammonium is already toxic at very small concentrations. Therefore, water bodies with an ammonium concentration of 1 mg/l are not suitable for fishing. Therefore, the discharge values that must be met are very low.
Why monitor ammonia?
Ammonia is used both as a reagent and as a measurement parameter in several areas of water and wastewater treatment.
- To monitor natural ammonia in source water.
- During chloramine disinfection, ammonia is combined with chlorine to treat drinking water and maintain a more permanent residue in the distribution system.
- Sometimes ammonia is used to control pH values, for example in the pharmaceutical industry.
- Ammonia is widely monitored in wastewater nitrification and denitrification processes.
Although generally harmless at low concentrations, ammonia at high concentrations can cause damage and pose a health risk. Therefore, ammonia levels must be properly monitored and maintained.
Ammonia & ammonium water sensor
The ammonium water sensor is an ion selective electrode (ISE) that measures charged ammonium ions in water. With some calculations, the sensor is also able to measure the ammonia concentration in the water.
The working principle of the ammonium ion selective electrode is based on the principle of diffusion potential. When the ISE is in contact with the solution to be measured, ammonium ions will preferentially pass through the selective membrane into the internal electrolyte due to the high selectivity of the selective membrane for ammonium ions. On both sides of the selective membrane, due to the different concentration of ammonium ions, a potential difference, i.e. the diffusion potential, is generated. There is a logarithmic relationship between the potential of the electrode and the concentration of ammonium ions in the solution to be measured, and the concentration of ammonium ions is quantified by measuring the potential.
Applications
- Water quality analysis: Determination of ammonium ion content in surface water, groundwater, wastewater and other water bodies.
- Soil analysis: Determination of ammonium and nitrogen content in soil.
- Food analysis: Determination of ammonium content in food.
- Biochemical research: for studying the metabolic process of ammonium ion in organisms.
- Industrial production: to monitor the concentration of ammonium ion in industrial production process.
Ammonia and ammonium
Ammonia is a colorless gas with a strong irritating odor. It is soluble in water and forms ammonia (NH₄OH). Ammonia is an important chemical raw material used in the production of fertilizers, explosives, and plastics.
Ammonium is an ion, consisting of one nitrogen atom and four hydrogen atoms. It is positively charged and can therefore combine with negatively charged ions to form a salt. Ammonium ions are an important nutrient in the soil and an important source of nutrition for many plants and animals.
Some of the common reactions in which ammonium and ammonia interconvert are listed below.
- Ammonia Hydrolysis: Ammonia in water dissociates into ammonium ions and hydroxide ions:
NH₃ + H₂O ⇌ NH₄⁺ + OH- - Dissolution of Ammonium Salt: When an ammonium salt is dissolved in water, it releases ammonium ions and the corresponding anions:
NH₄Cl dissolved in water → NH₄⁺ + Cl- - Synthesis of ammonia: Ammonia can be prepared by the synthesis of nitrogen and hydrogen:
N₂ + 3 H₂ ⇌ 2 NH₃ - Combustion of ammonia: Combustion of ammonia in air produces nitrogen and water:
4 NH₃ + 5 O₂ → 4 NO + 6 H₂O