What is dissolved oxygen(DO)?
Dissolved oxygen (DO) is a measure of how much oxygen is dissolved in water or other liquids. It is an important parameter in assessing water quality because it has an impact on the organisms living in the water body. The amount of dissolved oxygen in a stream or lake can tell us a lot about its water quality. High or low DO levels can harm aquatic organisms and affect water quality.

In scientific terms, dissolved oxygen is the level of free, non-combined oxygen present in water or other liquids. Uncomplexed oxygen or free oxygen (O2) is oxygen that is not bound to any other element. Dissolved oxygen is the presence of these free oxygen molecules in the water. The bonded oxygen molecules (H2O) in water are present in compounds and are not counted in the dissolved oxygen level. As you can imagine, free oxygen molecules dissolve in water in a manner very similar to how salt or sugar dissolves when stirred.
Dissolved oxygen units are usually expressed in parts per million (ppm) or micrograms per liter (mg/L) concentrations. Concentrations can also be expressed as percent saturation, where saturation is the maximum amount of oxygen that can theoretically be dissolved in water at a given pressure and temperature.
Dissolved oxygen in water
Dissolved oxygen is essential for many life forms, including fish, invertebrates, bacteria and plants. These organisms use oxygen in respiration, similar to organisms on land. Fish and crustaceans obtain oxygen for respiration through their gills, while plants and phytoplankton require dissolved oxygen for respiration when light is not available for photosynthesis. The amount of dissolved oxygen required varies from organism to organism. Bottom feeders, crabs, oysters and worms require minimal oxygen (1-6 mg/L), while shallow water fish require higher oxygen levels (4-15 mg/L).
Microorganisms such as bacteria and fungi also require dissolved oxygen. These organisms use dissolved oxygen to break down organic matter at the bottom of the water column. Microbial decomposition is an important contributor to nutrient cycling. However, if there is an excess of decaying organic matter (from dying algae and other organisms), oxygen at lower water levels will be used up more quickly in water bodies with infrequent or no turnover (also called stratification).
All aquatic animals need dissolved oxygen in order to respire. When excess organic material (e.g., macroalgal blooms) is decomposed by microorganisms, hypoxia (lack of oxygen) or anoxia (lack of oxygen) may occur. During this decomposition process, dissolved oxygen in the water is consumed. Low oxygen levels often occur at the bottom of the water column and affect organisms living in the sediment. In some water bodies, dissolved oxygen levels fluctuate periodically and seasonally, even as part of the natural daily ecology of aquatic resources. As dissolved oxygen levels decline, some sensitive animals may leave, decline in health or even die. The above reasons prove why dissolved oxygen is important in water.
Temperature and dissolved oxygen
Dissolved oxygen concentration in surface water is affected by temperature and has a seasonal and daily cycle. Cold water can hold more dissolved oxygen than warm water. In winter and early spring, when water temperatures are cooler, dissolved oxygen concentrations are higher. Dissolved oxygen concentrations tend to be lower in summer and fall, when water temperatures are warmer.
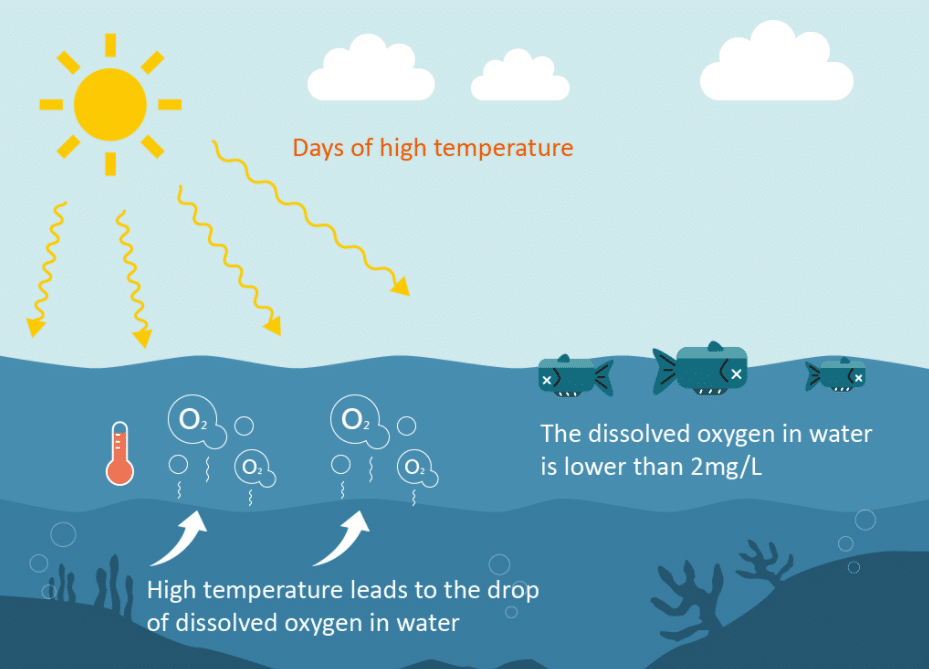
All forms of aquatic life use dissolved oxygen in surface water; therefore, this component is often measured to assess the “health” of lakes and streams. Oxygen enters streams from atmospheric and groundwater emissions. However, the contribution of oxygen in groundwater discharge is significant, but only in areas where groundwater is an important component of streamflow, such as in areas of glacial deposition. Photosynthesis is the primary process affecting the dissolved oxygen/temperature relationship; in turn, water clarity, light intensity and duration affect the rate of photosynthesis.
| Temperature(C) | CsDissolved oxygen(mg/L) | Temperature(C) | CsDissolved oxygen(mg/L) |
| 0 | 14.64 | 18 | 9.46 |
| 1 | 14.22 | 19 | 9.27 |
| 2 | 13.82 | 20 | 9.08 |
| 3 | 13.44 | 21 | 8.90 |
| 4 | 13.09 | 22 | 8.73 |
| 5 | 12.74 | 23 | 8.57 |
| 6 | 12.42 | 24 | 8.41 |
| 7 | 12.11 | 25 | 8.25 |
| 8 | 11.81 | 26 | 8.11 |
| 9 | 11.53 | 27 | 7.96 |
| 10 | 11.26 | 28 | 7.82 |
| 11 | 11.01 | 29 | 7.69 |
| 12 | 10.77 | 30 | 7.56 |
| 13 | 10.53 | 31 | 7.43 |
| 14 | 10.30 | 32 | 7.30 |
| 15 | 10.08 | 33 | 7.18 |
| 16 | 9.86 | 34 | 7.07 |
| 17 | 9.66 | 35 | 6.95 |
Dissolved oxygen measurement
Dissolved oxygen is considered an important indicator of water quality because it is a direct indicator of the ability of aquatic resources to support aquatic life. Dissolved oxygen levels are measured using a calibrated water quality probe meter, usually in combination with temperature and pH measurements. While each organism has its own dissolved oxygen tolerance range, in general, DO levels below 3 milligrams per liter (mg/L) are of concern, and water below 1 mg/L is considered hypoxic and usually lifeless.
Field and laboratory instruments for measuring dissolved oxygen have been around for a long time. As the figure shows, modern meters are small and highly electronic. They still use a probe located at the end of the cable. Dissolved oxygen is temperature dependent (inversely related), so the meter must be properly calibrated before each use.

Dissolved oxygen reading interpretation (mg/L)
0-2 mg/L: not enough oxygen to sustain life
2-4 mg/L: Only a few fish and insects can survive
4-7 mg/L: Acceptable for warm water fish
7-11 mg/L: ideal for most stream fish, including cold-water fish
For percent saturation.
Below 60%: poor; water too hot or bacteria depleting dissolved oxygen
60-79%: acceptable for most aquatic organisms
80-125%: very suitable for most aquatic organisms
112% or more: too high and may be dangerous to fish
More articles on dissolved oxygen:
Why is water quality important?
Main water quality indicators
What is salinity?
What is pH in water test?
