pH electrode working principle is based on electrochemical processes that allow the measurement of a solution’s hydrogen ion concentration, which is crucial for determining its pH value. pH electrodes are essential tools in chemistry, biology, and various industrial applications where accurate pH monitoring is necessary.
Principle of Working of pH Electrodes
pH electrodes work on the basis of the electrochemical Nernst Equation, which measures the concentration of hydrogen ions in a solution by a change in the potential difference to derive the pH value. Simply put, a pH electrode creates a potential difference between its interior and exterior by interacting with hydrogen ions in solution, and this potential difference is proportional to the acidity or alkalinity of the solution. Specifically, a pH electrode consists of a sensitive glass membrane and a reference electrode system:
- Sensitive glass membrane
The key component of a pH electrode is the glass membrane, which is selectively permeable to hydrogen ions. When the electrode is immersed in solution, hydrogen ions in solution react with the outer surface of the glass membrane, while the inner side of the glass membrane (in contact with a known buffer) also interacts with hydrogen ions. The difference in hydrogen ion concentration between the two sides creates a potential difference between the two sides of the glass membrane. - Reference electrode
The reference electrode provides a constant potential as a reference which does not vary with solution. The reference electrode is usually filled with a potassium chloride (KCl) solution and is in contact with the test solution through a porous junction. The function of the reference electrode is to provide a stable reference potential that allows the pH electrode to accurately measure the pH of the solution. - Potential measurement and conversion
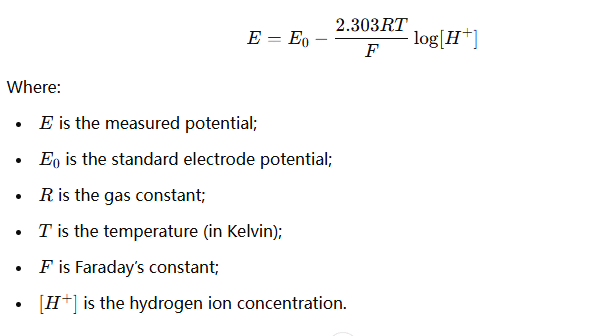
When the electrode is in solution, the pH meter measures the potential difference between the glass electrode and the reference electrode. This potential difference is closely related to the concentration of hydrogen ions in the solution, according to the Nernst equation:
Main Characteristics of pH Electrodes
- High sensitivity: The glass membrane senses very small changes in hydrogen ion concentration.
- Wide applicability: Suitable for pH measurement of liquids, semi-solids and even dissolved gases.
- Versatility: A wide range of designs, such as standard, flat, tip and miniature electrodes, are available to meet the needs of different applications.
Types of pH Electrodes
Classification by Function
(1) Composite electrode
Integrated measurement electrode and reference electrode, easy to use.
Widely used in laboratory, industrial on-line measurement and portable instruments.
Features: no need to configure a separate reference electrode, easy to operate.
(2) Single electrode
Contains only the measuring electrode and needs to be used with a separate reference electrode.
Features: Suitable for special occasions or measurement environments that require more flexible configurations.
Classification by Glass Membrane Characteristics
(1) General purpose pH electrode
Suitable for neutral or weak acid and alkali solutions.
The pH measurement range is usually 0-14 and the temperature range is 0-60°C.
(2) Strong alkaline electrode
Designed for highly alkaline solutions, suitable for pH measurement greater than 10.
Adopts special anti-alkaline glass membrane to avoid sodium ion error of ordinary glass membrane.
(3) Strong acid electrode
Used for the measurement of strong acidic solution, can withstand high concentration of acid environment.
(4) High temperature electrode
Adopting high temperature resistant glass film, suitable for high temperature environment (e.g. up to 130°C).
Commonly used in food processing, chemical and pharmaceutical industries.
Classification by Application
(1) Laboratory electrodes(e-201 Lab Glass pH Electrode)
Designed for high-precision measurements under laboratory conditions.
Usually equipped with a slender glass body suitable for insertion into beakers or test tubes.


(2) Industrial electrodes(PHK Industrial Digital Water pH Sensor)
Designed for on-line continuous monitoring, suitable for production environment.
Characteristics: corrosion-resistant, anti-pollution, long-term stability.
Often with automatic temperature compensation (ATC) function.


(3) Portable electrodes
Designed to be lightweight and easy to use, used in conjunction with portable pH meters.
Commonly used for on-site sampling and rapid testing.
(4) Miniature electrodes
Suitable for measurements in tiny samples or small volume containers.
Smaller diameter and higher sensitivity.
(5) Special purpose electrodes
Stabbing electrode: Used for solid or semi-solid samples such as food and soil.
Plane electrode: Suitable for measuring surface pH, such as skin, paper, etc.
Flow-through electrode: suitable for on-line measurement of pipeline fluids.
Classification by Shell Material
(1) Glass shell electrode
Commonly used in laboratories, high sensitivity, but fragile.
(2) Plastic shell electrode
Durable, drop-proof, suitable for portable equipment or industrial environment.
(3) Titanium shell electrode
High strength, corrosion resistant, suitable for harsh industrial environments.
Classification by Type of Reference Solution
(1) Filled electrode
The reference solution can be replenished to extend the life of the electrode.
Suitable for long-term use, but requires regular maintenance.
(2) Sealed electrode
The reference solution is sealed and cannot be replenished.
Advantages: maintenance-free, easy to use.
Classification by Interface
(1) Ceramic interface electrode
The most common, suitable for most applications.
(2) Sliding interface electrode
Reduces interfacial contamination and is suitable for highly contaminated solutions.
(3) Polytetrafluoroethylene (PTFE) interface electrode
Highly resistant to contamination, suitable for high viscosity or particle-containing samples.
(4) Open interface electrode
No risk of clogging, suitable for extremely complex samples (e.g. sewage, mud).
Ph Installation Method
Immersion Installation
Application scenario: Open liquid pool or sink.
Installation method: Mount the electrode on a bracket or fixture, ensuring that the electrode is completely submerged in the liquid.
Installation Depth: The electrode sensing head (glass membrane) must be immersed in the liquid to avoid exposure of the catchment interface. Avoid direct contact with the vessel wall or strong stirring device to prevent damage to the electrode.
Pipeline Installation
Application scenario: On-line monitoring of piping in closed systems.
Installation method:
Fix the electrode in the pipe flow channel through the flange or threaded interface.
Ensure that the electrode head is in contact with the flowing liquid and that there is no interference from air bubbles.
The mounting position is usually at the side or top of the horizontal pipe to prevent clogging by sediments.
Flow-through Installation
Application scenario: Small flow rate sample measurement.
Installation method:
Install the electrode in the flow-through cell or measurement unit, ensuring that the liquid can flow through the electrode sensor head sufficiently.
Maintain a certain flow rate (usually 0.3-0.8m/s) to avoid measurement errors caused by too fast or too slow flow.
Puncture Mounting
Application scenario: pH measurement of semi-solid, food or soil.
Installation method:
Pierce the electrode tip directly into the sample to ensure that the electrode is in full contact with the sample.
Avoid excessive force to prevent damage to the electrode.
Factors Affecting pH Electrode Performance
To ensure the accuracy of pH electrode measurements, several factors need to be attended to:
- Calibration: Periodic calibration with standard buffers (e.g. pH 4, 7 and 10) is required.
- Temperature: Temperature affects the potential difference of the electrode, so many pH meters are equipped with automatic temperature compensation (ATC).
- Contamination: Contaminants on the surface of the glass membrane can affect the accuracy of the electrode and require regular cleaning.
- Storage: Proper storage of pH electrodes (e.g., in a storage solution) will prevent drying and damage.
Maintenance and Care of pH Electrodes
Proper maintenance is essential to ensure long-term stable operation of the pH electrode:
After use, always rinse the electrode with distilled water and store in the recommended storage solution (avoid storing in pure water).
Clean the electrode regularly, especially when working with samples containing oils, proteins or sediments.
Avoid impact or severe friction on the glass membrane during use to prevent damage.
Summary
pH electrodes are precision instruments based on electrochemical principles that accurately calculate the pH value of a solution by measuring the potential difference in the concentration of hydrogen ions. Whether in the water treatment, pharmaceutical, food industry, or environmental monitoring fields, pH electrodes play an irreplaceable role. Understanding its working principle and proper calibration and maintenance can ensure its efficient and accurate pH measurement, providing important data for process control and quality management in various industries.
Apure not only provides ph electrodes, but also water quality analyzers for other parameters, not only that, but also flow and level measuring instruments, temperature and pressure measuring instruments. Please contact us if you need any further information.
