Resistivity of Water (Resistivity of Water) is an important indicator of the electrical properties of a body of water, measuring the ability of water to impede the flow of electric current. It has a wide range of applications in physics, electrochemistry and environmental sciences, and is particularly important in water quality analysis, industrial processes and the safety and security of power systems. Water itself is not a conductor of electricity, however, it can conduct electricity when dissolved with conductive substances such as ions.
What is Resistivity?
Resistivity is the ability of a substance to impede the flow of an electric current and is usually measured in ohm-meters (Ω-m). The resistivity of water reflects the ability of the ions in the water to conduct an electric current. Pure water (e.g., deionized water) has a higher resistivity, meaning that it is a greater impediment to the flow of electricity. In contrast, when salts or other ions are dissolved in the water, its resistivity decreases significantly.
Common Water Resistivity Values
The following are “typical” resistivity values for different bodies of water measured at 20°C (68°F):
- Sea water: 0.2 Ω-m (equivalent to a conductivity of about 5 S/m)
- Drinking water: 2-50 kΩ-cm (corresponding to a conductivity of about 20-500 μS/cm)
- Deionized water: 1-10 MΩ-cm (equivalent to approx. 0.1-1 μS/cm conductivity)
Relationship Between Resistivity and Conductivity
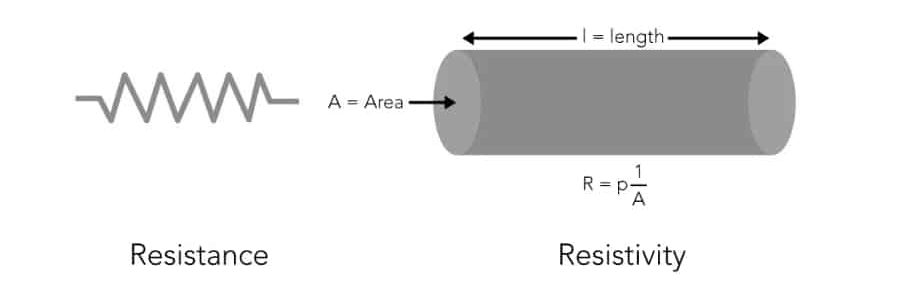
Resistivity and conductivity are inversely proportional, meaning that as the resistivity of a material increases, its conductivity decreases. Resistivity is an intrinsic property of a material and remains constant regardless of the material’s shape or size, whereas resistance depends on the material’s geometry.
Resistance measures a material’s opposition to the flow of electric current and can be calculated by multiplying the resistivity by the length of the material and dividing it by the material’s cross-sectional area.
The Importance of Resistivity in Water Quality Testing
1. Assessment of The Purity of Water
Resistivity can directly reflect the concentration of dissolved ions in water. Pure water has a high resistivity, which decreases when the water contains impurities or dissolved salts. Therefore, the higher the resistivity, the purer the water.
2. Contamination Detection
Pollutants in water, especially ionic pollutants (e.g. heavy metal ions, salts, etc.), can be quickly detected by resistivity measurement.
3. Industrial Water Treatment Control
In industry, resistivity is used to monitor and control water quality in cooling water, boiler water, and so on. Low resistivity can lead to problems such as corrosion and scaling of the system.
4. Environmental Monitoring
In environmental water quality monitoring, resistivity is an indicator for assessing the ionic content and mineralization of rivers, lakes and groundwater. Long-term monitoring of resistivity allows changes in water quality to be tracked, especially in water bodies contaminated by industry and agriculture.
What is the Resistivity Equation?

How do You Measure the Resistivity of Water?
Use of conductivity meters
A conductivity meter is an instrument designed to measure the conductivity of water. It assesses the ability of water to conduct electricity by immersing two electrodes in water and measuring the current between them. The conductivity can then be converted to resistivity according to the formula. Aprure A30 instrument is recommended for measuring the resistivity of water in conjunction with a conductivity meter.
Steps:
- Calibrate the instrument: Before taking measurements, calibrate the conductivity meter using a standard solution to ensure that the instrument reads accurately. A commonly used calibration solution is one with known conductivity
- Measuring water conductivity: The conductivity probe (with two electrodes) is immersed in a sample of water and held steady until the instrument displays a conductivity reading. The unit of conductivity is usually microsiemens/cm.

Temperature Correction
Temperature has a large effect on the conductivity of water, which normally increases with temperature. Therefore, temperature compensation is required during measurement to ensure accurate results. Conductivity meters usually have an automatic temperature compensation function that can normalize the measurement results to a specific temperature (usually 25°C).

Ensure clean water sample handling
In order to obtain accurate resistivity values, you need to ensure that the conductivity probe and vessel are clean during measurement. Any contaminants or ionic residues may affect the measurement results, especially in measurements of high resistivity water such as deionized or ultrapure water.
Calibration and maintenance
Calibrate the instrument and clean the electrodes regularly to ensure the reliability and accuracy of the measurement results. For high resistivity waters such as ultrapure water, specialized measurement electrodes may be required to improve the sensitivity of the measurement.
Factors Affecting Resistivity
- Concentration of dissolved ions: Determines the electrical conductivity of water; the more ions, the lower the resistivity.
- Temperature: The higher the temperature, the lower the resistivity.
- Water purity: Pure water has high resistivity, polluted or mineralized water has low resistivity.
- pH: Extremely acidic or alkaline water has a lower resistivity.
- Gas dissolution: Dissolution of gases such as CO₂ reduces resistivity.
- Pressure: Resistivity may be slightly reduced under high pressure.
- Organic and Suspended Matter: Indirectly affects the movement of ions in water.
Why is Resistivity Measured Instead of Resistance?
Resistivity is measured instead of resistance because resistivity is an inherent property of water, independent of the shape and size of the water sample. Resistance, on the other hand, can be affected by sample length and cross-sectional area, making it difficult to standardize and compare. Resistivity accurately reflects the purity and ionic content of water and is suitable for water quality analysis under different conditions.
Summary
The resistivity of water as an important physical property is widely used in water quality monitoring, industrial control, and electrical safety. It not only reflects the purity of water, but also provides valuable insight into conditions such as ion concentrations in water, temperature changes, and so on. Although there are many factors to consider when measuring the resistivity of water, such as temperature, dissolved ions, etc., accurate measurement methods and advanced instrumentation allow for a comprehensive assessment and control of the characteristics of a body of water.
As water treatment technology and testing equipment continue to advance, Apure water quality instrumentation measurements will play an even more important role in the environmental, industrial and power sectors. Welcome to contact us to customize exclusive water treatment solutions.
