Fluoride Electrode
Apure’s fluoride meter is one of the optional ion-specific electrodes (ISE) compatible with Apure’s multi-parameter water testing equipment. The fluoride ISE is sensitive to the concentration of fluoride ions in aqueous solutions. The solid-state sensor does not contain any liquid, so it can be stored dry and has a shelf life of more than 1 year.
Ion selective electrodes are part of a group of relatively simple and inexpensive analytical tools, often referred to as sensors or electrodes. Fluoride ion selective electrodes fall into this category and can be used to elucidate the basic principles of ISE.
Fluoride ISE is used to measure the concentration and activity of fluoride in aqueous solutions. Its preferred fields of application are drinking water, bone, cement, fish protein, glass, phosphate minerals and toothpaste analysis. In addition, it is even possible to measure the concentration of aluminum ions without specific electrodes. By fluorination of ISE, the addition of sodium fluoride solution precipitates aluminum into aluminum fluoride.
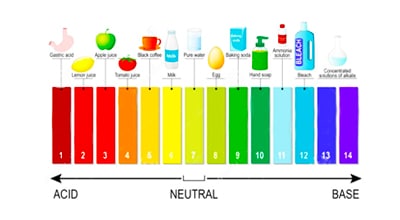
ISE is the preferred method for fluoride testing because there is less interference than other methods, although the electrode is still subject to interference from hydroxide (OH-) ions of a similar nature. Our fluoride meters are accurate to +/- 10% of the reading and, depending on the equipment you choose, can be deployed for long-term unattended water quality monitoring. Our ISE fluoride sensors are ideally suited to measure fluoride in water in the 0 – 1,000 mg/L (ppm) range.
What is a fluoride test?
A fluoride test is performed to investigate the amount of fluoride in water. Fluoride is a naturally occurring ionic compound (or salt) that is found in low levels in most bodies of water. The concentration of fluoride in water is influenced by the following factors: climate, geology, exposure to fluoride minerals, and the chemistry of the groundwater. Fluoride dissolves into water from the surrounding soil and rock. Some studies suggest that water temperature and hardness appear to affect fluoride toxicity, but more work needs to be done to strengthen this relationship.
In uncontaminated seawater, natural fluoride levels range from 1.3 to 1.4 mg/L. Levels are usually lower in estuaries due to dilution of freshwater, unless fluoride contamination occurs upstream. It is important to measure fluoride in water because fluoride is toxic to humans and aquatic life. Fluoride accumulates in the hard tissues of fish and shellfish, and then enters the food chain when the organisms are consumed.
Marine organisms can even accumulate fluoride in the surrounding sea level, and this accumulation increases significantly when higher levels of fluoride are present. Biomagnification occurs at about 1 order of magnitude per level as fluoride moves up the food chain.
Why measure fluoride levels in water?
One may want to monitor the level of fluoride in water to determine the environmental level of fluoride in the water body. Once a baseline level is known, continuing to measure fluoride in water will allow any contamination to be identified by any spikes in fluoride concentration. Measuring fluoride in water close to industrial areas, such as aluminum smelters, is necessary to carefully monitor fluoride levels in drinking water produced by water treatment plants. High concentrations are dangerous to humans and in extreme cases can lead to dental fluorosis or bone fluorosis.
Principle of fluoride ion selective electrode
Fluoride ion selective electrode, an electrochemical sensor for measuring the concentration of fluoride ions in solution. It consists of a conductive substrate (usually a lanthanide fluoride single crystal) and an internal reference electrode. The conductive substrate responds selectively to fluoride ions, while the internal reference electrode provides a stable reference potential.
When the fluoride ion digital electrode comes into contact with a solution, the fluoride ions in the solution react with the conductive matrix, resulting in a change in the electrode potential. The magnitude of the potential change is proportional to the logarithm of the concentration of fluoride ions in the solution. By measuring the change in potential, the fluoride ion concentration in solution can be determined.