pH is one of the most important indicators of water quality, which directly affects the chemical properties and biocompatibility of water. Controlling pH is critical in the water treatment process, especially in the treatment of pool water, industrial water and drinking water. High pH levels not only lead to water quality problems but can also cause damage to equipment and piping.
What’s ph?
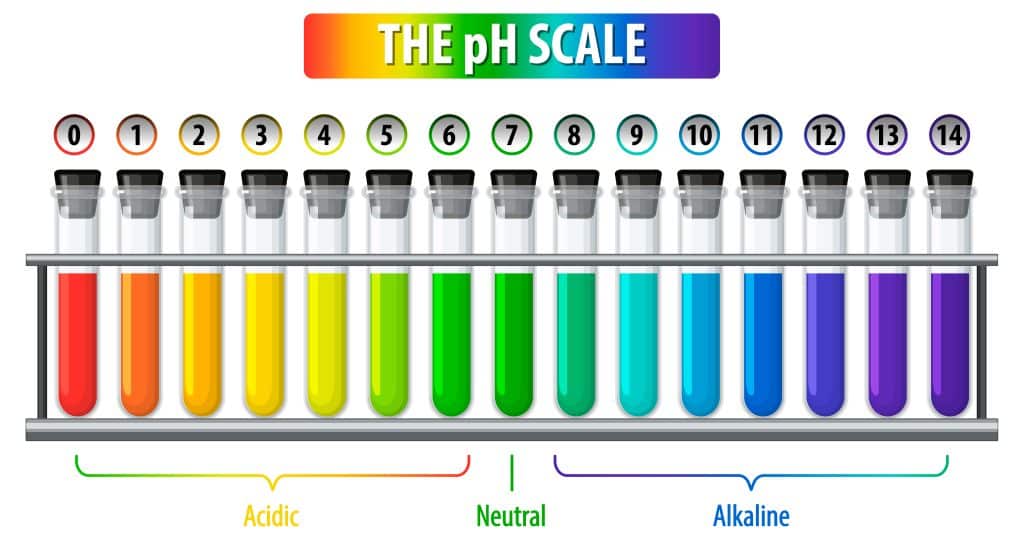
pH is a measure of the hydrogen ion concentration in a solution. It is a scale used to specify the acidity or basicity (alkalinity) of an aqueous solution. The pH scale ranges from 0 to 14, with 7 being neutral, below 7 being acidic, and above 7 being basic (or alkaline).
Measurement of pH
- pH 0-6: Acidic (e.g., lemon juice, vinegar)
- pH 7: Neutral (e.g., pure water)
- pH 8-14: Basic or alkaline (e.g., baking soda, soap)
The pH of a solution affects chemical reactions, biological processes, and the behavior of molecules in the solution. It’s an important parameter in chemistry, biology, medicine, agriculture, and environmental science.
Why lower the pH?
Adaptation to biological environment: Many organisms have requirements for growth and reproduction in specific pH ranges. For example, freshwater fish are typically adapted to water bodies with a pH between 6.5 and 8.5.
Water treatment requirements: In many water treatment processes, specific chemical reactions or precipitations require specific pH conditions. For example, in purified water treatment, pH adjustments can facilitate the precipitation or removal of impurities.
Preventing corrosion: The acidity or alkalinity of water has a significant impact on the corrosion of pipes and equipment. By controlling pH, corrosion damage to metal pipes and equipment can be reduced or prevented.
Improve water quality: Excessive pH may cause water to taste bitter, while proper adjustment can improve taste.
Chemical process control: In industrial processes, many chemical reactions need to be carried out under specific pH conditions to ensure product quality and reaction efficiency.
Effects of high pH
- Corrosion risk: High pH water may accelerate corrosion of metal piping and equipment, especially with prolonged exposure, which can lead to damage to piping and higher maintenance costs.
- Skin and mucous membrane irritation: High pH water may cause irritation or drying of the skin and mucous membranes, especially for people with sensitive skin.
- Mineral absorption problems: Extremely high or low pH levels may affect the body’s absorption of certain minerals, which can affect the body’s nutrient intake.
How do I test the pH in my water?
1. pH test paper
Dip the test paper into the water sample to be tested, remove the paper when it is sufficiently wet, and compare the color of the paper to the color card provided to determine the approximate pH range of the water. pH test paper is a simple and cost-effective method of estimating the pH value of water by the change in color of the paper.

2. pH sensor

A pH sensor uses an electrode to measure the concentration of hydrogen ions in a water sample to determine the pH value. Dip the electrode of the pH meter into the water sample, wait a few seconds until the reading stabilizes, and then read the pH value on the display.
3. Optical sensors
Optical sensors can infer pH by measuring the optical properties of certain chemical indicators (e.g. color, absorbance, etc.). This method is commonly used in specific industrial and environmental monitoring applications.
4. Electrochemical sensors
These sensors utilize an electrochemical reaction to measure pH in water samples, similar to traditional pH electrodes, but with greater sensitivity and stability.
5. Automated online monitoring system
An automated online monitoring system periodically measures the pH of a water sample through an electrode and sends the data to a centralized control system or logger. Installing and calibrating an online pH monitoring system ensures that the system takes pH measurements at predetermined intervals and with accuracy.

Caveats
- When selecting a pH test method, consider the accuracy requirements of the measurement and the environment in which it will be used.
- Calibrate pH instruments before use to ensure accuracy.
- Select the appropriate pH test method and equipment based on the type and chemical composition of the water sample being tested.
- Always follow safety procedures when handling chemicals and instruments.
How do you lower the ph in all kinds of water?
Drinking water
- Use of appropriate water filters: Some water filters adjust the pH of the water to make it more suitable for drinking. For example, some filters lower the pH of water by adding an acidic medium, such as carbon dioxide.
- Adding lemon juice or vinegar: Lemon juice or vinegar are common acids that can be used to adjust the pH of drinking water. However, it should be used in controlled amounts to avoid overdose.

Pool water
- Use of acidic chemicals: Pool-specific pH adjusters (e.g. hydrochloric acid or sulfuric acid) can quickly lower the pH of pool water.
- Adding carbon dioxide: Carbon dioxide can be used to steadily lower the pH of pool water through a CO2 injection system or by using a CO2 buffer, suitable for long-term control.

Cluster box water
- Use an aquarium pH regulator: Commercially available pH regulators can adjust the pH of the water precisely according to the aquarium needs to ensure the health of fish and aquatic plants.
- Adding or replacing water: Replacing a portion of the water or adding new water regularly can help stabilize the pH of your aquarium, especially if the water has a high pH level.

Water for plants
Acidic fertilizers: Some acidic fertilizers can help lower the pH of the soil when watered and are suitable for use on plants that require an acidic environment (e.g. rhododendrons).
Use an acidic water source: Tap water in some areas may be slightly acidic, making it suitable for use on plants requiring a low pH environment.
Ion exchange resin
Certain specific types of ion exchange resins can remove cations from water and release hydrogen ions, thereby lowering the pH of the water. This method is commonly used in industrial and water treatment facilities.

Reverse osmosis
Reverse osmosis is a physical method of removing dissolved salts and other impurities from water through a semi-permeable membrane filter, which may slightly lower the pH of the water.

Summary
Lowering the pH in water is a vital process for maintaining the health of both the environment and the equipment used in various applications. By understanding the methods available—from chemical additives to natural solutions and advanced automated systems—you can choose the most appropriate approach for your needs. Regular monitoring and adjustment are key to ensuring optimal water quality.
Apure not only offers a comprehensive range of water quality parameter testers but also specializes in a wide range of measurement equipment for a variety of application scenarios. Our product line includes high-precision flow meter equipment, level measurement equipment, temperature measurement, and ozone generator equipment to meet various industrial and commercial needs. Whether you are looking for an accurate water quality analysis solution or need an efficient flow control and monitoring system, please feel free to contact us for professional support and quality products.
